167(a)) by confirming the load has been exposed to the prescribed physical situations. This permits companies to couple adherence to sterilization cycle parameters by using a load observe to determine thermal lethality, therefore specifically confirming sterility and substituting for the sterility check.
So, Briefly, if you need to adhere to current Great Manufacturing Methods, you must have an outstanding assurance technique in position which could contend with each of the above details.
cGMP is really a secondary messenger in phototransduction in the attention. During the photoreceptors on the mammalian eye, the presence of light activates phosphodiesterase, which degrades cGMP.
Whilst the most important big difference is the usage of essentially the most existing benchmarks, you will find other noteworthy variances among GMP and cGMP.
The cGMP signaling pathway plays a job inside the regulation of neuroplasticity, a location of desire in comprehension the pathophysiology of main depressive problem (MDD).[thirteen] The cGMP signaling pathway inside the Mind operates as a second messenger method, amplifying neurotransmitter alerts, influencing gene expression and neuronal purpose.
suggests anyone or organizational ingredient specified through the company being liable for the duties associated with high-quality Handle.
The CGMP regulations for finished pharmaceuticals require the retention cgmp vs gmp of cleansing and use logs for non-dedicated tools, but no identical necessity exists for retaining what are intended to be rapid reference
When you have issues for your Agency that issued The present doc please Get in touch with the company immediately.
IRIS manual for applicants - How to build and post scientific purposes, for industry and unique applicants
FDA's procedure validation guidance now recommends an item lifecycle technique. The emphasis for demonstrating validated procedures is placed on the producer’s procedure style and development research in addition to its demonstration of reproducibility at scale, a target which has always been envisioned.
one µm pore sizing rated filters (see Faine 1982). Compendial microbiological take a look at techniques commonly used in association with upstream biotechnology and pharmaceutical output are certainly not capable of detecting this kind of microorganisms. No matter if this apparently unusual contamination hazard could be additional popular is unidentified, and we're sharing this information so that companies can think about regardless of whether this hazard could possibly be applicable for their operations.
Controlled by a variety of corporations and organizations, which include countrywide park authorities and camping associations.
Manufacturing facilities and laboratories with controlled environments, adhering to demanding cleanliness and safety expectations.
The that you're connecting for the official Site and that any information you offer is encrypted and transmitted click here securely.
 Scott Baio Then & Now!
Scott Baio Then & Now! Bradley Pierce Then & Now!
Bradley Pierce Then & Now! Barry Watson Then & Now!
Barry Watson Then & Now!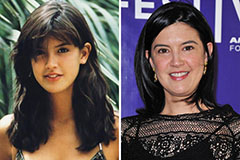 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Justine Bateman Then & Now!
Justine Bateman Then & Now!